need help?
[email protected]ICU 8'' Portable Medical Patient Monitor Vital Sign ECG NIBP RESP TEMP SPO2 PR
$194.83 $324.72
- Product Code: Unbranded
- Availability: 351
- Price in reward points: 28189
- 1000 Units in Stock
- All returns accepted:Returns Accepted
- Gain:2.5mm/mV, 5.0mm/mV, 10mm/mV, 20mm/mV
- SpO2::Measuring Range:0 ~ 100 %
- MPN:Does Not Apply
- Lead Mode:Lead Mode:5 Leads ( R, L, F, N, C or RA, LA, LL, RL,V)
- Condition:New
- Refund will be given as:Money back or replacement (buyer's choice)
- Return shipping will be paid by:Seller
- Number of Channels:5 Leads ( R, L, F, N, C or RA, LA, LL, RL,V)
- Model:Portable patient monitor
- Temperature::Measuring and Alarm Range:0 ~ 50 C
- Country/Region of Manufacture:China
- Heart Rate:15-300BPM(Adult) 15-350BPM(Neonatal)
- RESP:Adult 0 ~ 120 rpm,Neo/Ped 0 ~ 150 rpm
- Monitor Parameters:Blood Pressure - NIBP
- Features:Portable Patient Monitor
- Expiration Date:Brand New
- Screen Dimensions:8 inch
- Brand:Unbranded
- Restocking Fee:No
- Intended Use/Discipline:Cardiology
- warranty:2 years
- Technology:Digital
- Suitable for:adult, pediatric and neonatal patient
- NIBP:Method:Oscillometric
- Dispaly:8 inch TFT color screen
- Item must be returned within:30 Days
Description:
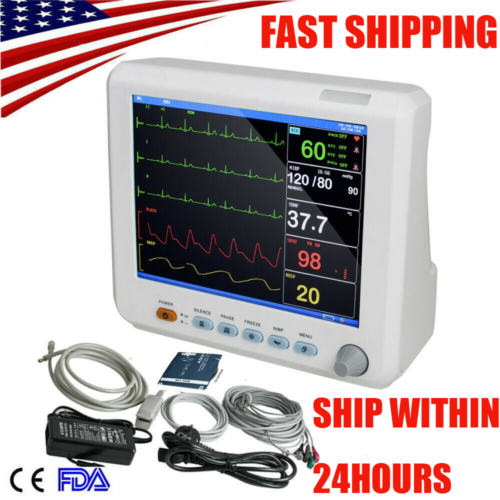
Portable Patient Monitor is adaptable to adult, pediatric and neonatal usage. It can monitor vital signals as ECG, Respiratory Rate, SpO2, NIBP, TEMP and IBP. It integrates parameter measuring modules, display and recorder in one device, featuring in compactness, lightweight and portability. Replaceable built-in battery facilitates transportation of patient. Large high-resolution display provides clear view of 5 waveforms and full monitoring parameters.
Features:
8 inch TFT color screen
Exquisite design suitable to moving operation
Various interfaces: standard screen, trend screen, oxyCRG screen, NIBP list screen, big front screen.
Real-time S-T segment analysis, pace-maker detection.
Built-in rechargeable lithium battery, convenient for patient moving.
Suitable for adult, pediatric and neonatal patient
Standard Configuration:
SpO2, PR, NIBP, ECG, RESP, TEMP
Technical Parameters:
ECG:
Lead Mode:5 Leads (
R, L, F, N, C or RA, LA, LL, RL,V)
Lead selection:I, II, III, avR, avL, avF, V,
Waveform:2 ch
Gain:2.5mm/mV, 5.0mm/mV, 10mm/mV, 20mm/mV
Heart Rate:15-300BPM(Adult) 15-350BPM(Neonatal)
Accuracy:± 1% or ± 1bpm,which great
Resolution:1 bpm
ST measurement Range:-2.0-+2.0mV
Sweep Speed:12.5 mm/s,25mm/s,50mm/s
Alarm:Available
Review:Available
RESP:
Method Impedance between R-F(RA-LL)
Measuring Range:Adult
0 ~ 120 rpm,Neo/Ped 0 ~ 150 rpm
Resolution:1 rpm
Accuracy:
±
2 rpm
NIBP:
Method:Oscillometric
Mode:Manual, Auto, STAT
Measuring and alarm range
Adult Mode
SYS:40 ~ 270 mmHg
DIA:10 ~ 215 mmHg
MEAN:20 ~ 235 mmHg
Pediatric Mode
SYS:40 ~ 200 mmHg
DIA:10 ~ 150 mmHg
MEAN:20 ~ 165 mmHg
Neonatal Mode
SYS:40 ~ 135 mmHg
DIA:10 ~ 100 mmHg
MEAN:20 ~ 110 mmHg
Resolution:1mmHg
Accuracy:+-5mmHg
SpO2:
Measuring Range:0 ~ 100 %
Alarm Range:0 ~ 100 %
Resolution:1 %
Accuracy:70% ~ 100%
±
2 %
Actualization interval:about 1 Sec.
Alarm Delay:10 Sec.
Pulse Rate :0~254bpm
Resolution:1bpm
Accuracy:
±
2bpm
Temperature:
Measuring and Alarm Range:0 ~ 50 C
Resolution:0.1C
Accuracy:0.1C
Actualization interval:about 1 Sec.
Average Time Constant:< 10 Sec.
Package List:
Patient monitor
Temperature probe
ECG lead cable
ECG electrode
Adult NIBP cuff
NIBP extension tube
Adult fingertip SpO2 probe
Power cord
User Manual
FDA Disclaimer:
The following FDA Disclaimer is required for all eBay listing in Healthcare category and is included for REFERENCE:
ARTG NO: 278908 ARTG Number: 278908
Statement: The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item. (The seller name: Helen Zhang,City:Beijing State: Beijing Country: China Telephone number: 86-18730611603)
This item has been cleaned and treated according to the manufacturer's instructions.
The Fingertip Pulse-Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 136606, and certified by FDA of United States (Premarket Submission Number (510K): K073454 Listing Number: D045684, K082641 Listing Number: D064765, K090671 Listing Number: D078664); and CE Approved, TUV of Europe (Cert.No. : G1 10 02 50972 013).
Payment information:
PayPal
only.
Please pay the money within 5 days after the auction ended.
Shipping method:
All items will be shipped within 1 business days.
For US Buyer About 2-7 business days!
We are not Responsible for your Customs duty.
Terms of sale
Warranty:
We guarantees new equipment for a period of
24 months
. Our company's obligation under this warranty is repairing or replacement.
Return Policy :
If you receive a defective item which you want to return, please contact us within 30 days from the day you receive the item. All return items must be returned with its original packaging and accessories.
Feedback:
Contact us first before leaving a negative, we will find a solution to meet your requirement and enhance the satisfaction.
About us
Quality First, Service Excellent, Global Trust!
We are the manufacturer and distributor of medical equipments for more than 10 years. If you are interested in the distribution work, please feel free to contact us.
We will reply to you within 24 hours. Please kindly wait for us.
Contact us
During the duty time ,we can give you a quick respond. If we are out of work ,please kindly be patient and considerable to give us a little more time ,not just leave the neutral or negative feedback without communication ,thank you for your cooperation !
Portable Patient Monitor is adaptable to adult, pediatric and neonatal usage. It can monitor vital signals as ECG, Respiratory Rate, SpO2, NIBP, TEMP and IBP. It integrates parameter measuring modules, display and recorder in one device, featuring in compactness, lightweight and portability. Replaceable built-in battery facilitates transportation of patient. Large high-resolution display provides clear view of 5 waveforms and full monitoring parameters.
Features:
8 inch TFT color screen
Exquisite design suitable to moving operation
Various interfaces: standard screen, trend screen, oxyCRG screen, NIBP list screen, big front screen.
Real-time S-T segment analysis, pace-maker detection.
Built-in rechargeable lithium battery, convenient for patient moving.
Suitable for adult, pediatric and neonatal patient
Standard Configuration:
SpO2, PR, NIBP, ECG, RESP, TEMP
Technical Parameters:
ECG:
Lead Mode:5 Leads (
R, L, F, N, C or RA, LA, LL, RL,V)
Lead selection:I, II, III, avR, avL, avF, V,
Waveform:2 ch
Gain:2.5mm/mV, 5.0mm/mV, 10mm/mV, 20mm/mV
Heart Rate:15-300BPM(Adult) 15-350BPM(Neonatal)
Accuracy:± 1% or ± 1bpm,which great
Resolution:1 bpm
ST measurement Range:-2.0-+2.0mV
Sweep Speed:12.5 mm/s,25mm/s,50mm/s
Alarm:Available
Review:Available
RESP:
Method Impedance between R-F(RA-LL)
Measuring Range:Adult
0 ~ 120 rpm,Neo/Ped 0 ~ 150 rpm
Resolution:1 rpm
Accuracy:
±
2 rpm
NIBP:
Method:Oscillometric
Mode:Manual, Auto, STAT
Measuring and alarm range
Adult Mode
SYS:40 ~ 270 mmHg
DIA:10 ~ 215 mmHg
MEAN:20 ~ 235 mmHg
Pediatric Mode
SYS:40 ~ 200 mmHg
DIA:10 ~ 150 mmHg
MEAN:20 ~ 165 mmHg
Neonatal Mode
SYS:40 ~ 135 mmHg
DIA:10 ~ 100 mmHg
MEAN:20 ~ 110 mmHg
Resolution:1mmHg
Accuracy:+-5mmHg
SpO2:
Measuring Range:0 ~ 100 %
Alarm Range:0 ~ 100 %
Resolution:1 %
Accuracy:70% ~ 100%
±
2 %
Actualization interval:about 1 Sec.
Alarm Delay:10 Sec.
Pulse Rate :0~254bpm
Resolution:1bpm
Accuracy:
±
2bpm
Temperature:
Measuring and Alarm Range:0 ~ 50 C
Resolution:0.1C
Accuracy:0.1C
Actualization interval:about 1 Sec.
Average Time Constant:< 10 Sec.
Package List:
Patient monitor
Temperature probe
ECG lead cable
ECG electrode
Adult NIBP cuff
NIBP extension tube
Adult fingertip SpO2 probe
Power cord
User Manual
FDA Disclaimer:
The following FDA Disclaimer is required for all eBay listing in Healthcare category and is included for REFERENCE:
ARTG NO: 278908 ARTG Number: 278908
Statement: The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item. (The seller name: Helen Zhang,City:Beijing State: Beijing Country: China Telephone number: 86-18730611603)
This item has been cleaned and treated according to the manufacturer's instructions.
The Fingertip Pulse-Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 136606, and certified by FDA of United States (Premarket Submission Number (510K): K073454 Listing Number: D045684, K082641 Listing Number: D064765, K090671 Listing Number: D078664); and CE Approved, TUV of Europe (Cert.No. : G1 10 02 50972 013).
Payment information:
PayPal
only.
Please pay the money within 5 days after the auction ended.
Shipping method:
All items will be shipped within 1 business days.
For US Buyer About 2-7 business days!
We are not Responsible for your Customs duty.
Terms of sale
Warranty:
We guarantees new equipment for a period of
24 months
. Our company's obligation under this warranty is repairing or replacement.
Return Policy :
If you receive a defective item which you want to return, please contact us within 30 days from the day you receive the item. All return items must be returned with its original packaging and accessories.
Feedback:
Contact us first before leaving a negative, we will find a solution to meet your requirement and enhance the satisfaction.
About us
Quality First, Service Excellent, Global Trust!
We are the manufacturer and distributor of medical equipments for more than 10 years. If you are interested in the distribution work, please feel free to contact us.
We will reply to you within 24 hours. Please kindly wait for us.
Contact us
During the duty time ,we can give you a quick respond. If we are out of work ,please kindly be patient and considerable to give us a little more time ,not just leave the neutral or negative feedback without communication ,thank you for your cooperation !